The Epidemiology goes viral session included the following presentations:
1. Laura Tonnetti: The Impact of Babesia Testing on Transfusion-transmitted Babesiosis
2. Wen-Jie Liu: Ten-year experience of mini-pool nucleic acid testing in blood donors in Taiwan
3. Brian Custer: Prevalence, Incidence, and Residual Risk of HIV, HBV, and HCV in the US Blood Supply 2015 – 2021, on Behalf of the US TTIMS Program
4. Eduard Grebe: HIV incidence in US first-time blood donors during 12-month and 3-month MSM deferral policy periods on behalf of the US TTIMS Program
5. Daniel Candotti: Ten years insight into HBV molecular epidemiology in infected blood donors in Dalian, Northeast China
MODERATORS: Dudi Levi, Dorte Holm
After the presentation, there was a questions and answers session, which is also included in the recording.
Abstract
Prevalence, incidence, and residual risk of HIV, HBV, and HCV in the US blood supply 2015–2021, on behalf of the US TTIMS program
E Notari1, J Haynes1, D Nelson1, S Stramer1, R Dodd1, D Kessler2, B Custer3, R Reik4, S Anderson5, H Yang5
1American Red Cross, Rockville, MD, 2New York Blood Center Enterprises, New York, NY, 3Vitalant Research Institute, San Francisco, CA, 4OneBlood, Orlando, FL, 5US Food and Drug Administration, Silver Spring, MD, United States
Background: Monitoring the results of infectious disease testing of the blood supply is important to assess safety and to evaluate the impact of changes in policies for assessing donor suitability.
Aims: To estimate the prevalence, incidence and residual infection risk for HIV, HBV, and HCV using the first 6 years (September 2015–September 2021) of the Transfusion-Transmitted Infections Monitoring System (TTIMS), which accounts for more than 50% of the US blood supply.
Methods: Four major blood collection organizations (American Red Cross, New York Blood Center Enterprises, One Blood and Vitalant) participated. Serologic and nucleic acid test (NAT) results for HIV, HBV and HCV were used with consensus criteria developed for positive results. Donor information, including basic demographics, was collected from routine records and data were assembled and managed centrally. Prevalences of positive results are presented per 100,000 donations; incidence was calculated per 100,000 person-years over 2-year periods among repeat donors. The likelihood of issuing a donation in the window period (residual risk, RR) was estimated from repeat donor incidence plus the assumed incidence for first-time donors, based upon the ratio of NAT yield among first-time and repeat donors. Residual risk from repeat donations and overall weighted donations are presented separately. A subgroup analysis of prevalence for the final year of the study (October 2020 to September 2021) was also performed.
Results: Overall, 41.7 million donations were reviewed and prevalence per 100,000 donations were between 1.6 and 3.0 for HIV, 5.3 to 6.6 for HBV and 9.1 to 20.2 for HCV; these were essentially stable for HIV and HBV but declined rapidly over the last 2 years for HCV. A total of 7,577,010 donations was evaluated for the final year of the study period with prevalence and 95% confidence intervals per 100,000 of 1.6 (1.4–1.9) for HIV, 5.3 (4.7–5.8) for HBV, and 9.1 (8.4–9.8) for HCV. Prevalence was higher among donations from males by a factor of 4.5 for HIV, 2.7 for HBV and 2.3 for HCV. HIV was most frequent among the 18–24 age group and for HBV and HCV, among the 25–39 age group. The lowest prevalence for HIV was seen among white donors, > than 1 race for HBV and Asian for HCV. Prevalence for all agents was highest among donations from the south US Census region. Incidence and RR declined for HIV and HCV but increased slightly for HBV. Overall incidence and RR estimates for the final 2-year period are shown in the Table; notably, the risk was less than 1 in 5 million for HIV and HCV and remained approximately 1 per million for HBV. Prior published TTIMS RR (per million) for HIV, HBV and HCV (Dec, 2017–Jul, 2019) were approximately 1:1.6, 1:1.0, and 1:2.0, respectively (Steele et al., Transfusion 2021).
PA18-L03 – Table 1
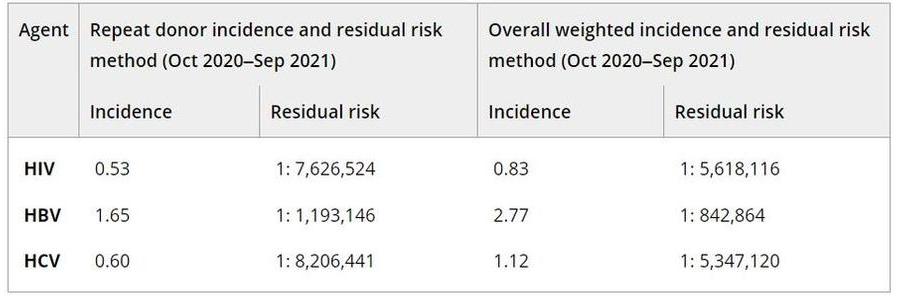
Summary/Conclusions: The prevalence and incidence of markers of transfusion-transmissible infections for more than 50% of the US blood supply are low, as is the risk of exposure for blood recipients. Prevalence over a 6-year period has been relatively stable or declining. This is particularly significant as the study covers the timeframe when deferral periods for HIV risk were reduced from permanent to 12 months, and then 3 months, and the early stages of the COVID-19 pandemic. These events were not associated with any apparent increase in risk to blood safety.

