The Change management - evidence based decisions session included the following presentations:
1. Barbee Whitaker: Modeling the Effect of an Individual-Risk Based Deferral Policy for Sexual Behaviors on Blood Donations in the US
2. Rachel Thorpe: Understanding preferences for advancing inclusion of trans and gender diverse people in blood donation in Australia
3. Stephen Thomas: Safety profile of plasma for fractionation donated in the United Kingdom, with respect to variant Creutzfeldt-Jakob Disease
4. Antoine Lewin: Risk of variant Creutzfeldt-Jakob Disease for the Canadian Blood Supply
5. Veronica Hoad: Is Dual Testing for hepatitis C necessary? Risk Modelling and Cost Effectiveness of removing hepatitis C antibody testing for Australian blood donors
6. Jennie Haw: Advancing inclusivity and equity for trans, nonbinary, Two-Spirit and other gender-diverse donors
MODERATORS: Eric Jansen and Marja-Kaisa Auvinen
After the presentation, there was a questions and answers session of about 5 minutes, which is also included in the recording.
Abstract
Modelling the effect of an individual-risk based deferral policy for sexual behaviours on blood donations in the US
B I Whitaker1, Y Huang1, D Gubernot1, A Eder2, R Forshee1
1CBER/OBPV, 2CBER/OBRR, US Food & Drug Administration, Silver Spring, MD, United States
Background: The blood donor deferral policy in the US to reduce the risk of HIV transmission by blood and blood components has evolved from an indefinite deferral of men who have sex with men (MSM) that was introduced in 1983, to a time-limited deferral after the last sexual contact, of 12 months in 2015 and 3 months in 2020. The UK and Canada have recently replaced the time-limited deferral for MSM with an individual-risk based approach for HIV sexual behavioural risk for all potential donors, regardless of sex/gender. FDA proposed draft recommendations in January 2023 that eliminate the time-based MSM deferral for MSM and related deferral for women who have sex with MSM, and instead ask all presenting blood donors the same risk-based screening questions and defer individuals who have had a new sexual partner or multiple sexual partners, and anal sex, in the past 3 months.
Aims: We calculated the effect of the individual-risk based deferrals on the current donor base, the 3-month donor deferral for MSM and the industry practice of deferral for pre-exposure prophylaxis (PrEP), and modelled the changes expected after implementation of a 3-month deferral for all presenting donors based on sexual behaviour with new and/or multiple partners and anal sex.
Methods: We developed a computational model to incorporate information about donors, sexual partners and sexual behaviours, including age- and sex-distributions and dependencies, assuming an independent relationship between the risk factors. In our model, both new or multiple partners and anal sexual behaviour must have been exhibited in the past 3 months to be considered a deferred donor. For new and multiple partners, we used the data for the past year to extrapolate for the last 3-month period. For anal sex, we applied the data for the past year and the past month to estimate possible maximum and minimum values for the 3-month period. Our model used 2021 blood donor age and sex distribution data from the US Transfusion Transmissible Infectious Disease Monitoring System (TTIMS) and survey data on US sexual behaviour from the National Health and Nutrition Examination Survey (NHANES 2015–2016), the National Survey of Family Growth (2015–2019), and the National Survey of Sexual Health and Behavior (2009).
Results: Our model estimates that an additional 0.35% to 1.16% of presenting donors would be deferred under the new recommendations (Table). We verified our results with pre-COVID-19 pandemic demographic data from TTIMS (2019).
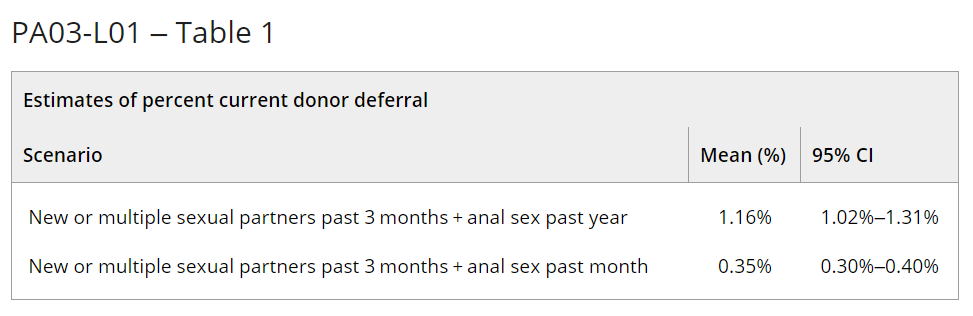
Summary/Conclusions: The model estimates that the effect of an individual-risk based deferral for sexual behaviour instead of the time-based deferrals for MSM is relatively small, and would only affect 0.35% to 1.16% of the current donor base. Our results are consistent with a 2018 Canadian donor survey (Caffrey, Transfusion Medicine, 2022) that estimated the donor loss of the individual-risk based deferrals as 0.7%.
The additional donor deferrals predicted in the US may be offset by gains from potential donors responding to a more inclusive donor assessment policy, although further studies are needed to assess the effect of the new policy on blood donation in the US.















