The Change management - evidence based decisions session included the following presentations:
1. Barbee Whitaker: Modeling the Effect of an Individual-Risk Based Deferral Policy for Sexual Behaviors on Blood Donations in the US
2. Rachel Thorpe: Understanding preferences for advancing inclusion of trans and gender diverse people in blood donation in Australia
3. Stephen Thomas: Safety profile of plasma for fractionation donated in the United Kingdom, with respect to variant Creutzfeldt-Jakob Disease
4. Antoine Lewin: Risk of variant Creutzfeldt-Jakob Disease for the Canadian Blood Supply
5. Veronica Hoad: Is Dual Testing for hepatitis C necessary? Risk Modelling and Cost Effectiveness of removing hepatitis C antibody testing for Australian blood donors
6. Jennie Haw: Advancing inclusivity and equity for trans, nonbinary, Two-Spirit and other gender-diverse donors
MODERATORS: Eric Jansen and Marja-Kaisa Auvinen
After the presentation, there was a questions and answers session of about 5 minutes, which is also included in the recording.
Abstract
Risk of variant Creutzfeldt-Jakob disease for the Canadian blood supply
A Pozzo di Borgo1,2, M Germain3, S O'Brien4, G Delage2, C Renaud2, A Lewin2,5
1Public Health, Université de Montréal, 2Medical Affairs and Innovation, Héma-Québec, Montreal, 3Medical Affairs and Innovation, Héma-Québec, Quebec, 4Epidemiology and Surveillance, Canadian Blood Services, Ottawa, 5Medicine and Health Science, Université de Sherbrooke, Sherbrooke, Canada
Background: Despite the >20-year absence of new transfusion-associated cases of variant Creutzfeldt-Jakob disease (vCJD), transfusion-transmission remains theoretically possible due to asymptomatic carriers. Blood services have introduced donor deferrals to mitigate this risk, but this measure limits the donor base.
Aims: To estimate the risk of a donation being contaminated with vCJD (“risk of vCJD”) in Canada should current vCJD deferral criteria be lifted.
Methods: The model used Bayesian networks and a Markov chain Monte Carlo method to simulate a cohort of 10 million blood donors observed between 2023 and 2120. The model accounted for several variables that may influence the risk of vCJD, including the number of “at-risk” donors (i.e., those with an extended travel/immigration history in Western Europe), PRNP genotype at codon 129, demographics, and the type of labile blood product donated. Donors were no longer considered at risk past the incubation period, as they should be deferred by pre-donation screening. Moreover, donors were assumed to be infectious only in the late phase of the incubation period. Separate models were developed for the two blood services in Canada, that is, Héma-Québec (HQ, which operates in Québec) and Canadian Blood Services (CBS, which operates in other provinces). Two estimates of vCJD prevalence were considered: a high estimate of 1 case in 2028 individuals derived from the Appendix-II study, and a low estimate of 1 case in 588,235 individuals derived from Garske to Ghani's stochastic model. Three scenario analyses were conducted to test the sensitivity of model outputs to assumptions on vCJD prevalence (i.e., high vs. low estimate); the presence or absence of a second wave; and the proportion of deferred at-risk donors, which was reduced by 20% relative to the base case in the optimistic scenario (Table 1).
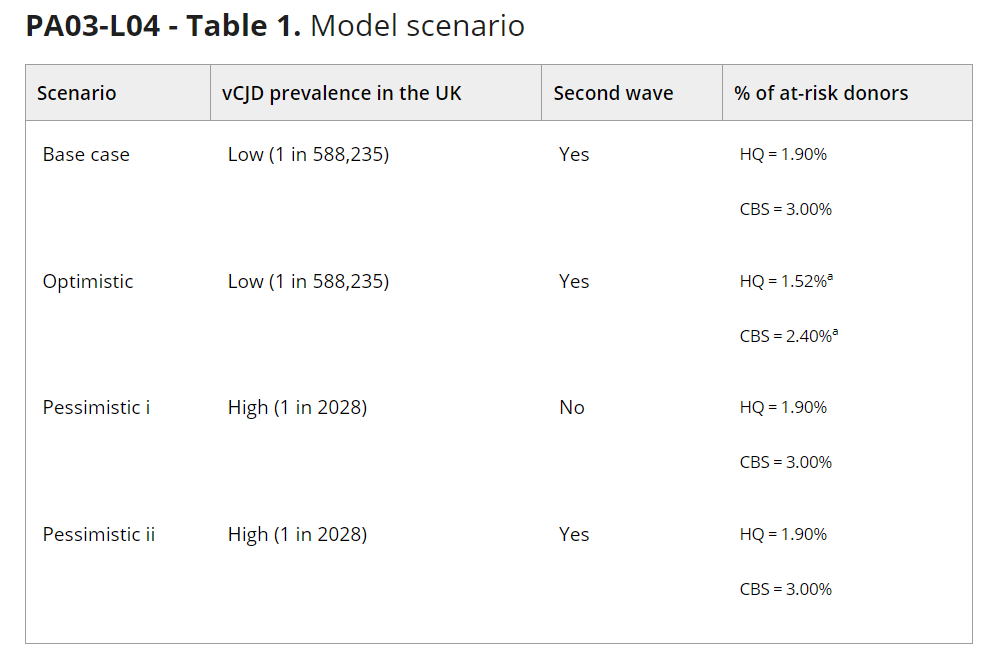
Results: In the base case, the simulated HQ cohort comprised 188,825 (1.9%) at-risk donors, and the CBS cohort comprised 324,313 (3.2%) such donors. Over 98 years of observation, no donors were predicted to be infectious at either blood service, and no donations would be contaminated. At HQ, the risk of vCJD was estimated at 0.0, or ≤1 in 294 million based on the upper bound of the 95% confidence interval (CI). At CBS, this risk was also estimated at 0.0, or ≤1 in 312 million based on the upper bound of the 95% CI. Similar results were obtained in the optimistic scenario, but not in pessimistic scenarios i (only for CBS) and ii. In pessimistic scenario i, at HQ, the risk of having a contaminated donation was estimated at 0.0, or ≤1 in 294 million based on the upper bound of the 95% CI. At CBS, this risk was ≤1 in 50 million based on the upper bound of the 95% CI. In pessimistic scenario ii, under the same assumption, at HQ, the risk of having a contaminated donation was estimated at ≤1 in 77 million based on the upper bound of the 95% CI. At CBS, this risk was ≤1 in 16 million based on the upper bound of the 95% CI.
Summary/Conclusions: Given that there are roughly 230,000 whole blood donations each year at Héma-Québec and 800,000 at CBS, the risk of having a vCJD-contaminated donation appears minimal in Canada (i.e., based on the 95% CI upper bound of the pessimistic scenario ii, 1 in 335 years and 1 in 20 years, respectively). These results thus support lifting current vCJD deferral criteria.















